AP Chemistry Free Response Questions
8 min read•july 17, 2020
J
Jordyn Haynes
J
Jordyn Haynes
AP Chemistry 🧪
269 resourcesSee Units
Overview of AP Chemistry FRQs
The written response section or Free Response Questions of the AP Chemistry is the second and final section of the AP Chemistry exam. The FRQs are designed to test the 6 major concepts covered throughout the AP Chemistry Course:
- Question and Method
- Models and Representations
- Representing Data and Phenomena
- Model Analysis
- Mathematical Routines
- Argumentation
The FRQs will also cover other important concepts such as experimental design and analysis of lab data and observations and will also look for a logical path towards your answer. There are 7 FRQs on the exam: 3 long answer questions and 4 short answer questions (we will break down the specifics of these two types of questions in a later section). All questions are multi-step questions and will test a variety of topics and skills learned throughout the AP Chemistry course.
For more info about these key concepts & skills straight from the College Board click here.
Exam Format
As you know, the FRQ section on the AP Chem exam is 7 questions long (3 long, 4 short answer questions). You will have 1 hour and 45 minutes to complete the section. Your score on this section equates to 50% of your total exam score. Here is a clip of Dylan Black, a chemistry streamer, going over the format of the exam as a whole.
Short Answer vs Long Answer Questions
The short answer questions are single or multi-part questions that are scored out of a total of 4 points. The long answer questions are always multi-part and are worth a total of 10 points. A good breakdown of time for these questions would be:
10 minutes for each SAQ and 16 minutes for each LAQ
Task Verbs
Here are the task verbs for the chemistry FRQ:
Image Courtesy of College Board
How to Ace the FRQ - Step by Step
Step 1 - Read the Entire Question
Read each part of the question from top to bottom or bottom to top. Make sure you understand what each part of the question is asking before moving on.
Step 2 - Break It Down
Figure out your plan of attack. Make a list of any given information and call to mind the skills you need to apply to answer the problem. Determine if calculations are needed or if you have to draw a diagram. Also, look out for questions asking you to “prove” or “justify” your answer and then think about any theoretical topics you’ve learned that will help you validate your answer.
Step 3 - Answer the Question
This is your time to shine! Use the information you have already organized to move through each problem. Apply all skills and perform all calculations. Make sure to answer all parts of the question and to justify your answer whether it be with a calculation, a rule/law, a trend, or theory. Make sure to reference both substances in your justification/explanation.
Step 4 - Double-Check
Make sure you’ve answered everything. Are your units and/or the number of significant figures correct? Have you missed anything? Are your calculations accurate? Walk through each step of the problem and connect it to how you got your answer.

Remember, you got this! Image Courtesy of GIPHY
AP Chemistry FRQ Tips
Concise and Clear is Key
Write with precision and confidence. Convey your answer clearly and accurately--nothing more, nothing less. Don’t distract your reader with rambling or long-winded explanations and make sure to use proper grammar.
Stay Consistent
Be careful to make sure all of your answers make logical sense and “add up” as you work from part 1 to the end of the problem
Cash In On Credit
In other words, maximize your credit on each problem. If a question has multiple answers make sure you select the best one. Always be sure to prove or justify your answer if applicable. You may get partial credit for any question so if you have any knowledge for any part of a question include it!
Words and Symbols
Sounds silly, but always be sure to accurately label your work. Make certain you correctly use terminology (ie. molecule, ion, mol, bond, atom, attraction, force, etc.) and symbols (ie. k, K, M, m, etc.)
Know Your Chemistry
It is super helpful to understand the major concepts and most important topics of Chemistry when trying to answer FRQs efficiently. Develop a good foundation with all of these but specifically intermolecular forces and periodic trends (as recommended by the College Board). Also, knowing your significant figures rules and conversion factors will boost your chances of getting every point possible!
Example FRQ - Short Answer (4 points)
A student is performing an experiment with NO2(g). The gas is in a rigid container, originally at 300. K and 2.0 atm. The student increases the temperature of the gas to 450 K.
Describe the effect of raising the temperature of NO2(g) on the movement of the NO2(g) molecules.
Calculate the pressure in the container at 450 K.
Use the Kinetic Molecular Theory to explain why the pressure of NO2(g) changes when the temperature is increased.
The student measures the actual pressure of NO2(g) in the container and sees it is less than the pressure predicted by the ideal gas law. Explain why this occurred.
Break Down
The question includes information about a sample of gas & there are 4 questions to answer.
What Do I Have to Do? Remember the Kinetic Molecular Theory and the Ideal Gas Law. Most gas questions can be broken down to those two concepts.
1A. As temperature increases, the gas molecules have a higher average amount of kinetic energy and move with a greater velocity. As a result, the pressure increases in a rigid container. The particles collide with the container walls with a greater amount of force.
1B. Use PV = nRT to calculate the new pressure at the higher temperature. All variables remain constant except pressure and temperature. Simplify down to
P1/T1 = P2/T2
(2.0 atm / 300. K) = ( P2 / 450. K)
P2 = 3.0 atm
1C. According to the Kinetic Molecular Theory, the average kinetic energy of a sample of gas particles is directly proportional to the kelvin temperature. When the temperature of the gas sample is increased, the average kinetic energy also increased, resulting in a greater velocity. This resulted in a greater pressure at the increased temperature, since the number of collisions the molecules have with the walls of the container increased.
1D. The real gas pressure is less than ideal gas pressure due to attractive forces between the NO2 gas particles. The NO2 molecule is polar and exerts attractive forces resulting in a lower gas pressure due to less molecules colliding with the container’s walls.
Scoring Guidelines
See below for a quick breakdown of each point.
Part A:
1 point comes from correctly relating temperature to gas motion.
Part B:
1 point comes from correctly calculating P2.
Part C:
1 point comes from correctly justifying the relationship between Kinetic Molecular Theory to pressure and temperature.
Part D:
1 point comes from correctly explaining the causes of “real” gas pressure.
Example FRQ - Long Answer (10 points)
Here is an example of the process described above for answering a free-response question. This is a long answer question, but the steps taken to answer all FRQs should be relatively the same. Begin by completing a thorough read-through of the entire question.
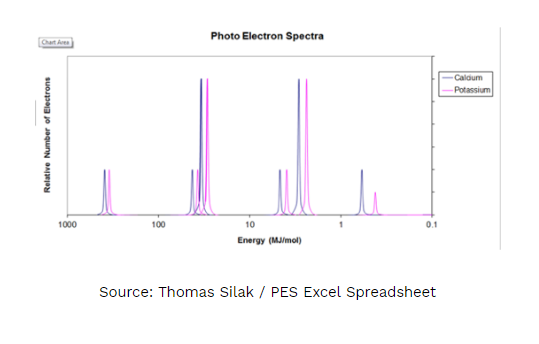
1. Answer the following questions regarding the photoelectron spectra for calcium and potassium.
A. On the PES spectra above write an X above calcium’s valence electrons. Justify your answer using Coulomb’s Law.
B. Explain WHY every peak for calcium is shifted slightly to the left of potassium.
C. Would the peaks for an ion of calcium (Ca2+) be shifted to the left, right or stay the same as the peaks of the calcium atom shown in the diagram? Justify your answer.
D. Would the photoelectron spectra of 39K and 40K be the same or different? If they were different, in what way(s)? If they were the same, why? Justify your answer.
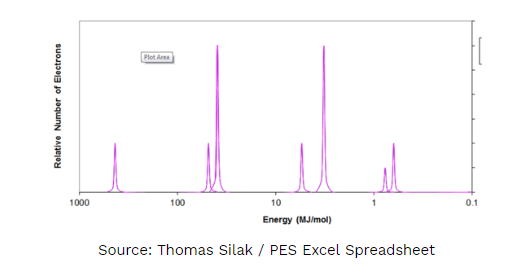
E. Identify the element shown above.
- Write the electron configuration.
- Explain to which energy level the shortest peak must (just to the right of 1.0 MJ/mol) must be assigned. Justify your answer.
Break Down
The question includes 2 PES graphs & there are 5 questions to answer.
What Do I Have to Do?
I have to draw an “X” above the peak that represents the valence electrons for calcium and use Coulomb’s Law to justify my answer.
1A. The ‘X’ should be above the purple peak just to the right of 1 MJ/mol. This peak represents the valence electrons for calcium. The height equals 2 electrons and the position at the lowest binding energy means that those electrons are held onto with the least amount of energy. Using Coulomb’s law, the valence electrons are the furthest away from the nucleus and have the weakest interaction. There is significant shielding between the valence electrons and the nucleus resulting in the lowest binding energy. As the distance increases the energy decreases (inverse square relationship).
1B. Calcium has a higher effective nuclear charge than potassium, as a result the electrons are more tightly held than potassium’s electrons. This results in every electron in calcium having a slightly higher binding energy than the electrons in potassium.
1C. Stay the same, however, the peak for the valence electrons (the rightmost peak) would be missing. Both the calcium ion and calcium atom have the same effective nuclear pull, therefore the binding energy would be the same.
1D. The PES spectra of the potassium isotopes would be the same. Both isotopes have the same number of protons, resulting in the same effective nuclear pull and the same binding energies for electrons in the electron clouds. Isotopes differ in number of neutrons which is irrelevant when discussing binding energies (ionization energies).
1E. Scandium
1Ea. 1s2 2s2 2p6 3s2 3p6 4s2 3d1
1Eb. 3d1 This peak only has 1 electron and is filled after 4s2. It has slightly higher binding energy suggesting that it is NOT a valence energy level electron, this puts it in energy level 3.
Calculations/Equations?
N/A
Scoring Guidelines
The exact source of points in an AP Chemistry FRQ differs on a problem to problem basis, but you will always get the points for the correct answer. However, the correct answer is not always the only point to scoop up for any given question. Points will often come from correct explanations, units, secondary calculations, or balancing an equation. You may also earn partial credit on FRQ problems for answers that follow the correct approach but have the wrong numeric answer or answers that have some but not all parts of the correct answer.
See below for a quick breakdown of each point.
Part A:
1 point comes from correctly naming the location of the “X” AND 1 point for using Coulomb’s Law (effective nuclear charge) to justify the answer. See why justification and proving your answer is important?
Part B:
1 point comes from correctly justifying the location of calcium’s peaks.
Part C:
1 point comes from correctly answering the question and 1 point for correctly justifying the answer.
Part D:
1 point comes from correctly answering the question and 1 point for correctly justifying the answer.
Part E:
1 point comes from correctly identifying the element.
Part Ea:
1 point comes from the correct electron configuration.
Part Eb:
1 point comes from the correct energy level and and for correctly justifying the answer.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
AP Chemistry Free Response Questions
- Overview of AP Chemistry FRQs
- Exam Format
- How to Ace the FRQ - Step by Step
- AP Chemistry FRQ Tips
- Concise and Clear is Key
- Stay Consistent
- Cash In On Credit
- Words and Symbols
- Know Your Chemistry
- Example FRQ - Short Answer (4 points)
- Break Down
- Scoring Guidelines
- Example FRQ - Long Answer (10 points)
- Break Down
- Scoring Guidelines
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

© 2023 Fiveable Inc. All rights reserved.