Dylan Black
Kanya Shah
Dalia Savy
Dylan Black
Kanya Shah
Dalia Savy
AP Chemistry 🧪
269 resourcesSee Units
Interactions between solvents and solutes can be represented with particle/molecule diagrams. Remember that the solute is the substance that is dissolved, while the solvent is the substance that does the dissolving.
Types of Solutions
Solutions in which water is the dissolving medium, or solvent, are called aqueous solutions💧.
- Any substance whose aqueous solution contains ions is called an electrolyte.
- Any substance that forms a solution containing no ions is a nonelectrolyte.
Electrolytes
Electrolytes are substances that would generate electricity in a solution. This is explained by the presence of ions in the solution, and ions can carry an electric current. If a substance is an electrolyte, it can be either strong or weak:
- Strong electrolytes are substances that completely dissolve in water into their constituent ions. To explain this, we say that strong electrolytes completely dissociate in an aqueous solution. To dissociate is to simply split up into the respective cations and anions.
- Strong electrolytes include soluble salts, strong acids, and strong bases.
- Soluble salts include sodium chloride (NaCl) and potassium chloride (KCl).
- The strong acids and bases that you have to know in this course are found in the table below.
- Let's take a look at the strong acid HCl as an example of a strong electrolyte. The dissolution of hydrochloric acid in water can be represented by the following: HCl (aq) + H2O (l) --> H+ (aq) + Cl- (aq). This reaction proceeds until there is no more HCl, meaning if you were to dissolve five moles of HCl in water, you would find that there are five moles of hydrogen ions and chloride ions in the end.
| Strong Acids | Strong Bases |
| HCl | CaOH |
| HBr | SrOH |
| HI | BaOH |
| HNO3 | any group 1 metal + OH- |
| H2SO4 | |
| HClO3 | |
| HClO4 |
- Weak electrolytes are substances that only partially dissociate in water and conduct electricity, but not as strongly as strong electrolytes. Since these partly dissociate in a solution of water, only some molecules would split up into their ions.
- Weak electrolytes include weak acids and weak bases.
- Acetic acid, CH₃COOH, is a weak acid and weak electrolyte. The dissolution of acetic acid in water can be represented by the following chemical equation: CH₃COOH (aq) + H2O (l) --> H+ (aq) + CH3COO- (aq). This reaction does not completely proceed. In other words, only some of the acetic acid will dissolve into hydrogen and acetate.
Nonelectrolytes are molecular compounds that do not conduct electricity since there are no ions present that can carry an electric current. An example would be sugar🍬.
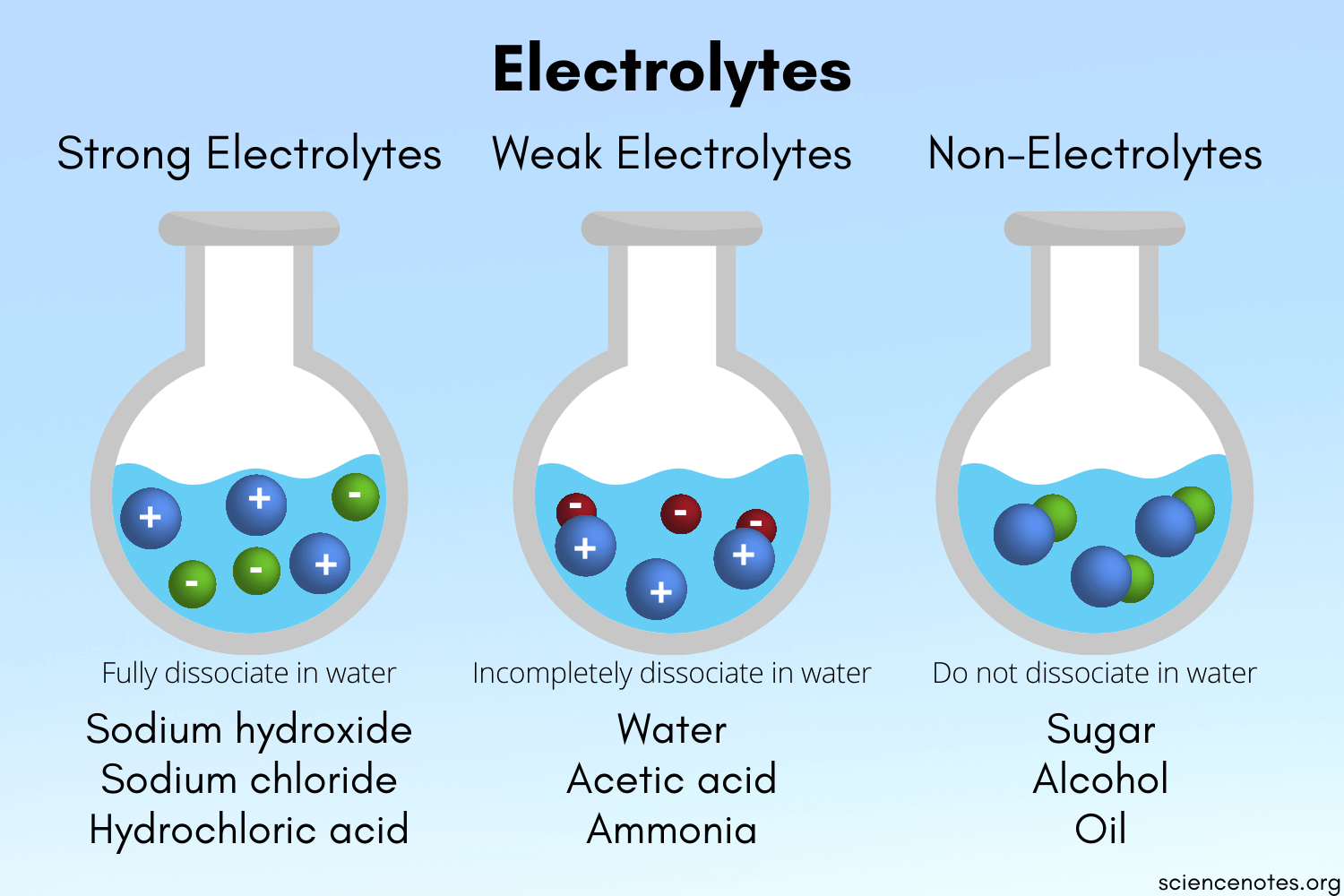
Image Courtesy of Science Notes; You can see in this diagram the varying dissociation of substances that make them either strong electrolytes, weak electrolytes, or nonelectrolytes.
Acids and Bases
The solubility of ionic substances is made possible by solvation, the process of a solvent dissolving a solute to form a solution.
Acids and bases are critical electrolytes as mentioned above. Acids are proton donors; they increase the concentration of H+ (aq). Bases, on the other hand, are proton acceptors; they increase the concentration of OH- (aq). Knowing the basic properties of these electrolytes can help you recognize compounds that are in certain solutions.
Acids and bases will be looked at further in both unit four about chemical reactions and unit eight which is all about them!
Visualizing Solutions
When knowing how different solutions act regarding electrolytes, acids, and bases, you can visualize how particles interact in a solution:
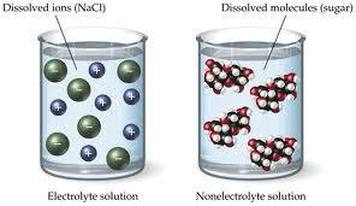
Image Courtesy of Kesley Putman
In the above images, you can see two dissolved substances, one being a strong electrolyte and the other being a non-electrolyte. Since the NaCl solution shows dissolved ions, we know that it will conduct electricity.
Colligative Properties
⚠️ According to the AP Chemistry Course Exam and Description provided by College Board, colligative properties will not be assessed on the AP exam. We are going to discuss them just in case your teacher goes over them though.
When discussing solutions, colligative properties are properties that depend upon the concentration of solute molecules or ions, but not upon the identity of the solute. For example, a solution of 6M NaCl will act a lot differently than simply pure water.
There are three main colligative properties, but you may also learn about osmotic pressure.
Vapor-Pressure Lowering
The vapor pressure of a solution is lower than the vapor pressure of a pure solvent. When a solute is dissolved in a solvent, you decrease the tendency for water molecules💧 to evaporate into the gas phase♨️. This means that there will be less gas above the surface of the liquid, leading to lower vapor pressure.
There is a formula for finding the exact new vapor pressure:
Raoult's Law: P1 = XP0, where P1 is the new vapor pressure, X is the mole fraction of the solute, and P0 is the initial vapor pressure.
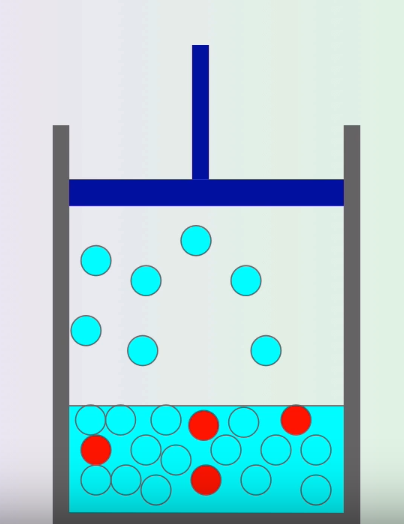
The red solute particles block gas from escaping, leading to a lower vapor pressure. Image Courtesy of MisterChemistry
Boiling-Point Elevation
The boiling point of a solution is higher than the boiling point of the pure solvent. Applying a non-volatile solution decreases the solution's vapor pressure by Raoult's Law. Then, the temperature must be increased🌡️ to return the vapor pressure to the pure solvent value. This means that as you add more and more solute, the temperature at which the solution boils increases⬆️.
A good example of this is when you make pasta🍝. Chefs around the world always recommend that you salt your water before you add pasta🧂, and while this of course is to add flavor, it also actually raises the boiling point of your water, making your pasta cook quicker!
This same principle is seen in chemistry with solutions. There is another formula to calculate the exact elevation.
ΔTb = km, where ΔTb is the change in boiling point, k is a specific constant, and m is the molality of the solute (molality is a measure of concentration that is mol solute/kg solvent).
Freezing-Point Depression
The same applies to freezing points of solutions. As the boiling point elevates, the freezing point depresses, or lowers. Therefore, the freezing point of a solution is lower than the freezing point of the pure solvent.
This means that, for example, a solvent may have a freezing point of 0 degrees Celsius without any solute, and then once you dissolve some solute, the freezing point will lower to -1.5 degrees Celsius.
A good example of this is if you live in the North, you are well accustomed to adding salt to ice in order to melt it❄️. The key reason for this is not a reaction occurring, but the freezing point of the ice decreasing, turning it back to a liquid.
Fun fact: The salt that melts ice on the streets is not NaCl! It's typically CaCl2 since it will split into more solute particles, thus decreasing the freezing point even more! NaCl splits up into two ions, whereas CaCl2 splits up into 3 ions.
The formula for freezing-point depression is very similar to boiling-point elevation; it is ΔTf = -km.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

© 2023 Fiveable Inc. All rights reserved.