Dylan Black
Jillian Holbrook
Dylan Black
Jillian Holbrook
AP Chemistry 🧪
269 resourcesSee Units
Welcome to the last section of Unit 8! This section is a relatively simple and short one all about buffer capacity, which helps us gauge the effectiveness of buffers. For a quick reminder, buffers are important because they are resistant to changes in pH.
However, buffers are not infinitely resistant. Eventually, the buffer will weaken and succumb to the acid/base being added. This is why, despite there being buffers in your bloodstream, chugging hydrochloric acid is a very bad idea. Seriously, don't do it!
Buffer capacity helps us see how much acid/base one can add until there is a significant change in pH.
Describing Buffer Capacity
As said by the Henderson-Hasselbalch equation, the pH of a buffer is defined by the ratio of the concentrations of the conjugate base to the acid, or in math terms [A-]/[HA]. The capacity of a buffer is determined by the magnitudes of these concentrations.
What do we mean when we refer to magnitude? Essentially, the magnitude of each concentration describes how large the concentrations are. A concentration of 5M would have a higher magnitude than a 0.5M solution. The more concentrated the acid and conjugate base, the stronger the buffer is at reducing pH changes! There is more acid and conjugate base to be resistant to strong acids/strong bases in a similar volume.
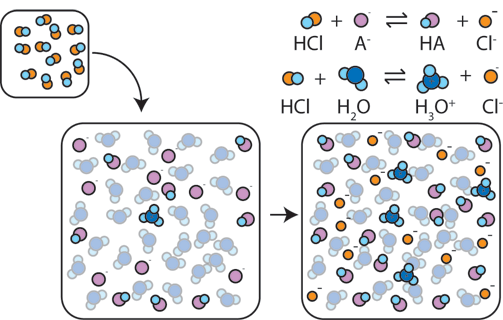
Image From ChemCollective
Example Problem: Identifying the Stronger Buffer
To practice applying buffer capacity, try thinking about two separate buffer systems, one with 5M acetic acid and 5M sodium acetate and another with 0.05M acetic acid and 0.05M sodium acetate. Because the ratios of the conjugate base to the acid are the same, both of these buffers will have the same pH (pH=4.74). However, our question asks us the following:
After HCl is added to each buffer system, the first one has a resulting pH of 4.74, and the second one has a resulting pH of 4.56. Which one has the better buffering capacity and why?
We can see that in the first buffer, the pH remained relatively unchanged, whereas, in the second, the pH dropped. Because the first system's pH remained constant, the first buffer is more effective at resisting pH changes and, therefore, has a better buffering capacity. The magnitude of the concentration of both the acid and the conjugate base is higher in the first buffer compared to the second, implying the first buffer will also have a stronger buffer capacity.
Practice Multiple Choice Questions
The College Board likes to use buffer capacity specifically on multiple choice questions because, unlike many other questions relating to buffers, questions about buffer capacity are very often qualitative. That means your answer will relate to some sort of non-numerical conclusion based on the information you are given.
They could ask you to identify the stronger buffer, like in the last question, or ask about a change in a system and how this may affect the buffer capacity.
Here is another example:
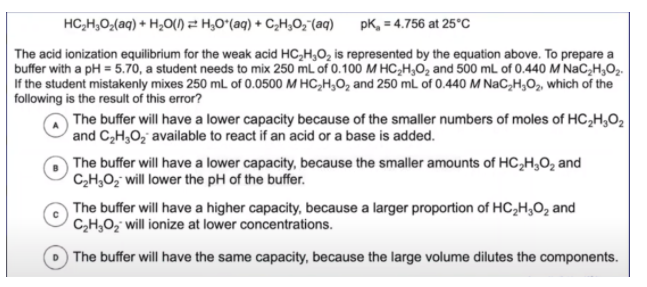
This problem gives us a BUNCH of information and can be really overwhelming at first. However, by breaking it down piece by piece, the problem is more approachable.
We know that a student is creating a buffer of acetic acid (CH3COOH, also stated as HC2H3O2) and sodium acetate since a weak acid mixes with its conjugate base. Now we can examine the numbers. The first set of numeric information indicates that the student wants to mix 250 mL of 0.100M acetic acid with 500 mL of 0.440M sodium acetate.
Yikes! The student makes an error. They choose an acid with half the concentration and a conjugate base with half of the volume. For acetic acid, the volume remains at 250mL, but the molarity is now 0.0500M. Meanwhile, the molarity of the sodium acetate stays at 0.440M, but the volume falls from 500mL to 250mL. The problem wants to know the ramifications of these mistakes.
Before looking at the answer choices, what changed? In both sets (the weak acid and the conjugate base), the number of moles is halved. Therefore, we will have half the number of moles of each species in our buffer.
Again, before looking at any answer choices, how is the buffer impacted? As we discussed earlier, a lower number of moles of each reactant in the buffer creates less resistance to changes in pH. In other words, the buffer capacity lowers.
Which answer choice matches that concept?

If we read the answer choices, we see that what we just described fits answer A. You may be tempted to pick B, but remember that buffer capacity is determined by the number of moles of the weak acid and the conjugate base.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

© 2023 Fiveable Inc. All rights reserved.